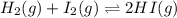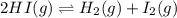Chemistry, 19.11.2019 17:31 mattydoug4818
Two experiments were performed involving the following equilibrium. the temperature was the same in both experiments. h2(g) + i2(g) 2hi(g) in experiment a, 1.0 m i2 and 1.0 m h2 were initially added to a flask and equilibrium was established. in experiment b, 2.0 m hi was initially added to a second flask and equilibrium was established. which of the following statements is always true about the equilibrium concentrations?
a.[h2] equals [hi] in experiment a.
b.[hi] equals 2[h2] in experiment a.
c.[hi] in experiment a equals [hi] in experiment b.
d.[hi] in experiment a equals 1/2[i2] in experiment b.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Two experiments were performed involving the following equilibrium. the temperature was the same in...
Questions


Physics, 06.12.2021 05:30


English, 06.12.2021 05:30

Mathematics, 06.12.2021 05:30

History, 06.12.2021 05:30




Law, 06.12.2021 05:30

History, 06.12.2021 05:30

Chemistry, 06.12.2021 05:30

English, 06.12.2021 05:30




Mathematics, 06.12.2021 05:30


Advanced Placement (AP), 06.12.2021 05:30

English, 06.12.2021 05:30