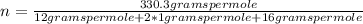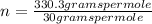Chemistry, 08.01.2020 22:31 jet0120996
The empirical formula for sucrose is ch2o. the molar mass of sucrose is 330.3 grams per mole. determine the molecular formula of sucrose.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
The empirical formula for sucrose is ch2o. the molar mass of sucrose is 330.3 grams per mole. determ...
Questions

Computers and Technology, 22.12.2020 18:50

Mathematics, 22.12.2020 18:50



English, 22.12.2020 18:50

Mathematics, 22.12.2020 18:50


Mathematics, 22.12.2020 18:50

Mathematics, 22.12.2020 18:50

Mathematics, 22.12.2020 18:50

English, 22.12.2020 18:50


Mathematics, 22.12.2020 18:50




Physics, 22.12.2020 18:50


Mathematics, 22.12.2020 18:50

Mathematics, 22.12.2020 18:50