Chemistry, 09.10.2019 04:30 richardgibson2005
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbon dioxide (co2), and water (h2o). a chemist carries out this reaction in a bomb calorimeter. the reaction causes the temperature of a bomb calorimeter to decrease by 0.985 k. the calorimeter has a mass of 1.500 kg and a specific heat of 2.52 j/g•k. what is the heat of reaction for this system?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbo...
Questions

Mathematics, 01.07.2019 03:30


History, 01.07.2019 03:30

History, 01.07.2019 03:30

Mathematics, 01.07.2019 03:30

History, 01.07.2019 03:30

History, 01.07.2019 03:30

Advanced Placement (AP), 01.07.2019 03:30

Mathematics, 01.07.2019 03:30


History, 01.07.2019 03:30

Chemistry, 01.07.2019 03:30







Mathematics, 01.07.2019 03:30

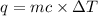
 = change in temperature = 0.985 K
= change in temperature = 0.985 K