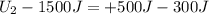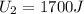Chemistry, 31.01.2020 22:43 jackie6852
In a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,500 j?
800 j
1,300 j
200 j
1,700 j

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

You know the right answer?
In a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the...
Questions

Biology, 06.07.2019 14:00


History, 06.07.2019 14:00

Mathematics, 06.07.2019 14:00









Geography, 06.07.2019 14:00

Health, 06.07.2019 14:00

Chemistry, 06.07.2019 14:00





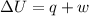
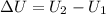 =Final energy-initial energy=Change in internal energy
=Final energy-initial energy=Change in internal energy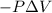 {Work done by the system is negative as the final volume is greater than initial volume}
{Work done by the system is negative as the final volume is greater than initial volume}