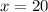Chemistry, 04.02.2020 15:50 applereams
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two isotopes, boron-10 and boron-11. determine the percent of each isotope of boron


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two...
Questions

Mathematics, 05.07.2019 03:00

Mathematics, 05.07.2019 03:00






History, 05.07.2019 03:00

Chemistry, 05.07.2019 03:00

Mathematics, 05.07.2019 03:00



Mathematics, 05.07.2019 03:00

History, 05.07.2019 03:00

Mathematics, 05.07.2019 03:00




Business, 05.07.2019 03:00

English, 05.07.2019 03:00

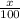
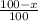
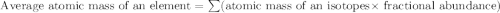
![10.8=\sum[(10)\times \frac{x}{100})+(11)\times \frac{100-x}{100}]]](/tpl/images/0500/8694/6044f.png)