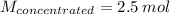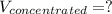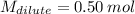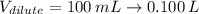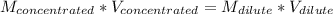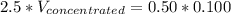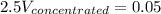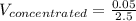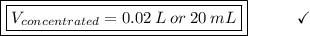Answer the question below. type your response in the space provided. what volume of a 2.5 m stock solution of acetic acid (hc2h3o2) is required to prepare 100.0 milliliters of a 0.50 m acetic acid solution? use the equation mconcentrated*vconcentrated = mdilute*vdilute.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Answer the question below. type your response in the space provided. what volume of a 2.5 m stock so...
Questions


Mathematics, 07.02.2021 08:10




Mathematics, 07.02.2021 08:10



Business, 07.02.2021 08:10



Chemistry, 07.02.2021 08:10

Social Studies, 07.02.2021 08:10

Mathematics, 07.02.2021 08:10



History, 07.02.2021 08:10

Mathematics, 07.02.2021 08:10


Mathematics, 07.02.2021 08:10