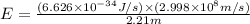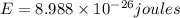Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s constant is 6.626 x 10-34 joule seconds, the speed of light is 2.998 x 108 m/s)
* 8.9886 x10-26 joules
*4.8844 x 10-42 joules second
* 1.9864 x 10-25 joules
* 1.4643 x 10-33 joules /second

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

You know the right answer?
Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s c...
Questions


Mathematics, 30.08.2019 04:20

Mathematics, 30.08.2019 04:20

Mathematics, 30.08.2019 04:20

Social Studies, 30.08.2019 04:20

Mathematics, 30.08.2019 04:20

Spanish, 30.08.2019 04:20

Chemistry, 30.08.2019 04:20

English, 30.08.2019 04:20

Physics, 30.08.2019 04:20


English, 30.08.2019 04:20




Mathematics, 30.08.2019 04:20


Social Studies, 30.08.2019 04:20


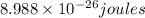
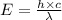
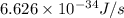
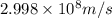
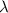 = wavelength = 2.21 m
= wavelength = 2.21 m