
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) + 2al(s)⟶ 2alcl3(aq) + 3cu(s) however, impurities and errors can occur easily. suppose that only 2.86 g of copper is produced. what is the percent yield of the reaction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) +...
Questions








Biology, 01.08.2019 11:00

Social Studies, 01.08.2019 11:00







Biology, 01.08.2019 11:00

Social Studies, 01.08.2019 11:00

Mathematics, 01.08.2019 11:00


Health, 01.08.2019 11:00

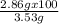 = 81 %
= 81 %

