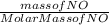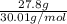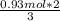Chemistry, 31.08.2019 18:10 jesh0975556
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxide (no)? 4nh3 + 6no → 5n2 + 6h2o the molar mass of ammonia is 17.0337 grams and that of nitric oxide is 30.01 grams.
a- 23.7 grams
b- 10.5 grams
c- 73.5 grams
d- 32.7 grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxid...
Questions

Mathematics, 18.10.2020 15:01


Health, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Spanish, 18.10.2020 15:01



Chemistry, 18.10.2020 15:01



Mathematics, 18.10.2020 15:01

Computers and Technology, 18.10.2020 15:01

Arts, 18.10.2020 15:01

English, 18.10.2020 15:01


English, 18.10.2020 15:01

History, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01