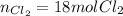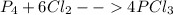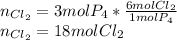Chemistry, 20.10.2019 12:50 Nathaliasmiles
For the reaction p4+6cl2 yields 4pcl3, determine how many moles of cl2 would be needed to react with 3 moles of p4 to entirely use up all phosphorous.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
For the reaction p4+6cl2 yields 4pcl3, determine how many moles of cl2 would be needed to react with...
Questions

Mathematics, 12.10.2019 13:50

History, 12.10.2019 13:50


Social Studies, 12.10.2019 13:50


Computers and Technology, 12.10.2019 13:50

Mathematics, 12.10.2019 13:50



Chemistry, 12.10.2019 13:50




Mathematics, 12.10.2019 13:50

Spanish, 12.10.2019 13:50




Health, 12.10.2019 13:50