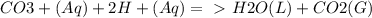Chemistry, 21.09.2019 08:50 yarrito20011307
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). the essential neutralization products are co2 and/or h2o. write net ionic equations to represent the neutralizing action of the following popular antacids:
-rolaids
-maalox
-tums
-milk of magnesia

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 23.06.2019 11:30
What makes up a film badge? layers of photographic film covered in black paper. an electrolyte paste spread across a plastic sheet. a thin film of salts and other chemicals on clear acetate. a panel of photographic film exposed to the air. ✓
Answers: 2
You know the right answer?
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). t...
Questions

Biology, 30.08.2019 10:20



Mathematics, 30.08.2019 10:20

Social Studies, 30.08.2019 10:20


Mathematics, 30.08.2019 10:20



Health, 30.08.2019 10:20


Chemistry, 30.08.2019 10:20



Social Studies, 30.08.2019 10:20


Computers and Technology, 30.08.2019 10:20

History, 30.08.2019 10:20