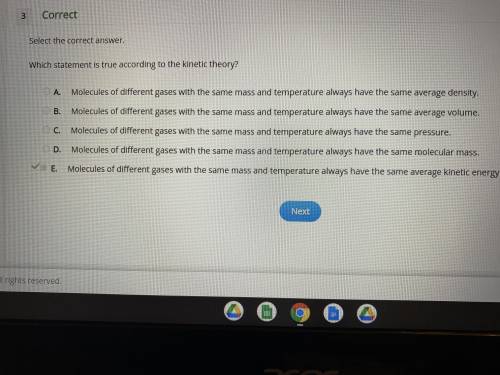
Which statement is true according to the kinetic theory?
molecules of different gases with th...

Chemistry, 28.12.2019 14:31 QueenNerdy889
Which statement is true according to the kinetic theory?
molecules of different gases with the same mass and temperature always have the same average density.
molecules of different gases with the same mass and temperature always have the same average volume.
molecules of different gases with the same mass and temperature always have the same pressure.
molecules of different gases with the same mass and temperature always have the same molecular mass.
molecules of different gases with the same mass and temperature always have the same average kinetic energy.
nextreset

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
Questions


Physics, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Physics, 16.12.2020 14:00

Chemistry, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

English, 16.12.2020 14:00


Geography, 16.12.2020 14:00

Mathematics, 16.12.2020 14:00

Arts, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

History, 16.12.2020 14:00

History, 16.12.2020 14:00

Arts, 16.12.2020 14:00

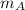 and
and 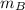 and velocity
and velocity 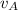 and
and 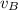 respectively. If
respectively. If 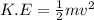 . Then it follows that
. Then it follows that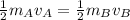 . Since the mass is the same it means the velocity of the two objects should be the same so that they have the same average kinetic energy.
. Since the mass is the same it means the velocity of the two objects should be the same so that they have the same average kinetic energy.