
Chemistry, 17.10.2019 16:50 lanaiheart7
Question 2
given the reaction, pcl3 + cl2 imported asset pcl5, if 3.00 moles of cl2 are used, then how many moles of pcl5 are made?
6.00 moles
5.00 moles
3.00 moles
1.00 mole
3 points save answer
question 3
given the balanced equation, fe2o3 + 3 co imported asset 2 fe + 3 co2, what is the molar ratio of co to co2?
3: 3
3: 1
3: 2
1: 3
3 points save answer
question 4
using the equation, 4fe + 3o2 imported asset 2fe2o3, if 96.0 g of oxygen react, what mass of iron was oxidized?
32.0 g
96.0 g
167 g
223 g
3 points save answer
question 5
using the equation, c5h12 + 8o2 imported asset 5co2 + 6h2o, if 10 moles of carbon dioxide are produced, how many moles of pentane (c5h12) would need to be supplied?
2 moles
0.5 mole
5 moles
10 moles
3 points

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
Question 2
given the reaction, pcl3 + cl2 imported asset pcl5, if 3.00 moles of cl2 are...
given the reaction, pcl3 + cl2 imported asset pcl5, if 3.00 moles of cl2 are...
Questions

Advanced Placement (AP), 26.12.2019 10:31








Mathematics, 26.12.2019 10:31






Biology, 26.12.2019 10:31




Mathematics, 26.12.2019 10:31

Mathematics, 26.12.2019 10:31

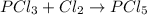
![PCl_5]](/tpl/images/0328/7661/93b9a.png)
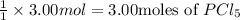
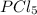 are made.
are made.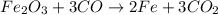
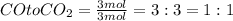
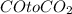 is 3:3.
is 3:3.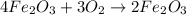
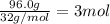
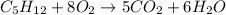
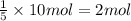 of pentane.
of pentane.

