
Aphoton has a wavelength of 6.2 meters. calculate the energy of the photon in joules. (planck's constant is 6.626 × 10-34 joule seconds, the speed of light is 2.998 × 108 m/s) 3.564 × 10-43 joules 3.204 × 10-26 joules 41.08 × 10-34 joules 13.702 × 10-42 joules
answer b

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

You know the right answer?
Aphoton has a wavelength of 6.2 meters. calculate the energy of the photon in joules. (planck's cons...
Questions

Chemistry, 05.07.2019 09:50

Social Studies, 05.07.2019 09:50

Chemistry, 05.07.2019 09:50

Chemistry, 05.07.2019 09:50

History, 05.07.2019 09:50

Business, 05.07.2019 09:50









Social Studies, 05.07.2019 09:50


Business, 05.07.2019 09:50

Social Studies, 05.07.2019 09:50

Biology, 05.07.2019 09:50

Biology, 05.07.2019 09:50

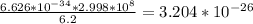 J. Hope this helps you!
J. Hope this helps you!

