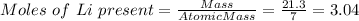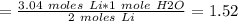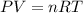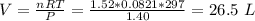Chemistry, 23.12.2019 22:31 terrysizemore666
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 297 kelvin and 1.40 atmospheres? show all of the work used to solve this problem. 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at...
Questions



Mathematics, 28.10.2020 17:10

Social Studies, 28.10.2020 17:10





Mathematics, 28.10.2020 17:10




Physics, 28.10.2020 17:10






English, 28.10.2020 17:10

Mathematics, 28.10.2020 17:10