
Chemistry, 02.09.2019 02:30 adkinsryan72
15 g of hydrogen reacts with 25 g of nitrogen. how much excess reagent will be left over when the reaction stops?
5.36 g of excess
7.23 g of excess
12.12 g of excess
19.27 g of excess
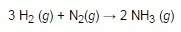

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
15 g of hydrogen reacts with 25 g of nitrogen. how much excess reagent will be left over when the re...
Questions

Computers and Technology, 04.02.2020 11:57

Mathematics, 04.02.2020 11:57


Mathematics, 04.02.2020 11:57

History, 04.02.2020 11:57


Mathematics, 04.02.2020 11:57







Mathematics, 04.02.2020 11:57

Social Studies, 04.02.2020 11:57

Mathematics, 04.02.2020 11:57

Mathematics, 04.02.2020 11:57





