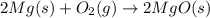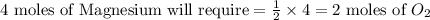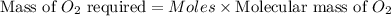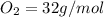Chemistry, 23.01.2020 14:31 blessed4628
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo).
2mg + o2 mc009-1.jpg 2mgo
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg?
2.00

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions



Mathematics, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50


Mathematics, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50

History, 26.02.2021 17:50


Mathematics, 26.02.2021 17:50


Mathematics, 26.02.2021 17:50

Mathematics, 26.02.2021 17:50

Biology, 26.02.2021 17:50


Social Studies, 26.02.2021 17:50


Mathematics, 26.02.2021 17:50

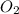 will be required to react completely with 4 moles of Mg.
will be required to react completely with 4 moles of Mg.