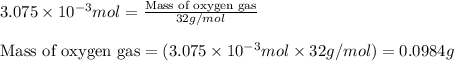Oxygen is produced by the reaction of sodium peroxide and water.
2na2o2(s) + 2h2o(l) > o2(g) + 4naoh(aq)
a. calculate the mass of na2o2 in grams needed to form 4.80g of oxygen.
b. how many grams of naoh are produced when 4.80 g of o2 is formed?
c. when 0.48g of na2o2 is dropped in water, how many grams of o2 are formed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Oxygen is produced by the reaction of sodium peroxide and water.
2na2o2(s) + 2h2o(l) &g...
2na2o2(s) + 2h2o(l) &g...
Questions

Biology, 05.05.2020 11:29

Mathematics, 05.05.2020 11:29

English, 05.05.2020 11:29

Mathematics, 05.05.2020 11:29



Mathematics, 05.05.2020 11:29

Mathematics, 05.05.2020 11:29


Mathematics, 05.05.2020 11:29


Mathematics, 05.05.2020 11:29

History, 05.05.2020 11:29




Arts, 05.05.2020 11:29



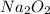 required is 23.4 grams
required is 23.4 grams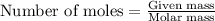 .....(2)
.....(2)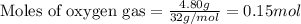
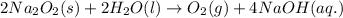
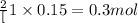 of
of 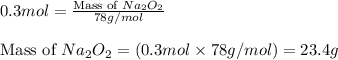
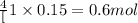 of NaOH will be produced.
of NaOH will be produced.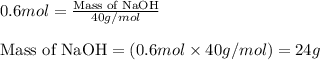
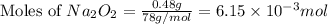
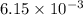 moles of
moles of 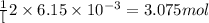 of oxygen gas
of oxygen gas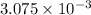 moles
moles