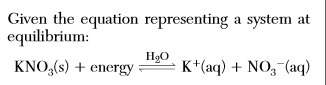Given the equation representing a system at equilibrium:
which change causes the equilibrium...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Questions

Mathematics, 29.07.2020 09:01

Geography, 29.07.2020 09:01

English, 29.07.2020 09:01

Mathematics, 29.07.2020 09:01



English, 29.07.2020 09:01


Mathematics, 29.07.2020 09:01

Health, 29.07.2020 09:01

Social Studies, 29.07.2020 09:01

Computers and Technology, 29.07.2020 09:01





Mathematics, 29.07.2020 09:01


Mathematics, 29.07.2020 09:01

Mathematics, 29.07.2020 09:01