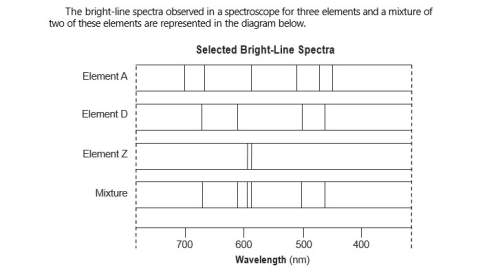
Describe, in terms of both electrons and energy states, how the light represented by
the spect...

Chemistry, 26.12.2019 06:31 perezshayla56
Describe, in terms of both electrons and energy states, how the light represented by
the spectral lines is produced.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Questions





Mathematics, 12.07.2021 21:40

Mathematics, 12.07.2021 21:40

Mathematics, 12.07.2021 21:40

Mathematics, 12.07.2021 21:40



Mathematics, 12.07.2021 21:50




Mathematics, 12.07.2021 21:50



English, 12.07.2021 21:50




