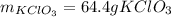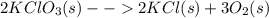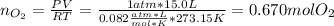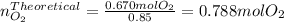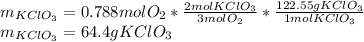Chemistry, 02.02.2020 21:52 tonimgreen17p6vqjq
The decomposition of potassium chlorate yields oxygen gas. if the yield is 85%, how many grams of kclo3 are needed to produce 15.0 l of o2 ?
2kclo3(s) -- > 2kcl(s) + 3 o2(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
The decomposition of potassium chlorate yields oxygen gas. if the yield is 85%, how many grams of kc...
Questions







Engineering, 28.09.2019 00:20


Engineering, 28.09.2019 00:20

Engineering, 28.09.2019 00:20

Engineering, 28.09.2019 00:20


Mathematics, 28.09.2019 00:20



Engineering, 28.09.2019 00:20