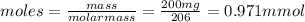Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers....

Chemistry, 04.02.2020 12:58 savannahvargas512
Asap
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet contains 200 mg of ibuprofen, and a typical adult dose is two tablets every six hours.
• determine the molar mass of ibuprofen. show all steps to find the answer.
• calculate the number of moles of ibuprofen in a single tablet. show all steps to find the answer.
• calculate the number of moles of ibuprofen that an adult would have taken if she took four doses of ibuprofen in one day. show all steps to find the answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1
You know the right answer?
Questions



Arts, 26.02.2021 18:20



Mathematics, 26.02.2021 18:20





Spanish, 26.02.2021 18:20


English, 26.02.2021 18:20

Computers and Technology, 26.02.2021 18:20


Health, 26.02.2021 18:20

Mathematics, 26.02.2021 18:20


Mathematics, 26.02.2021 18:20