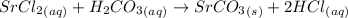Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Write a balanced net ionic equation for the following reaction: srcl2(aq) + h2co3(aq) → srco3(s) +...
Questions


Business, 06.05.2020 18:57

History, 06.05.2020 18:57



English, 06.05.2020 18:57


Computers and Technology, 06.05.2020 18:58




English, 06.05.2020 18:58




Mathematics, 06.05.2020 18:58


Mathematics, 06.05.2020 18:58

Mathematics, 06.05.2020 18:58