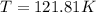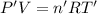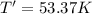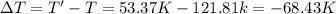Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm. if 0.713 mol ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of the gas mixture? the initial temperature of the gas is k.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Arigid, 2.50 l bottle contains 0.458 mol he. the pressure of the gas inside the bottle is 1.83 atm....
Questions

Mathematics, 18.08.2019 17:50


Mathematics, 18.08.2019 17:50


Mathematics, 18.08.2019 17:50

Mathematics, 18.08.2019 17:50

Biology, 18.08.2019 17:50


Mathematics, 18.08.2019 17:50


English, 18.08.2019 17:50




Biology, 18.08.2019 17:50

English, 18.08.2019 17:50

Geography, 18.08.2019 17:50

Mathematics, 18.08.2019 17:50

Mathematics, 18.08.2019 17:50

History, 18.08.2019 17:50

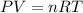 ( Ideal gas equation)
( Ideal gas equation)