
Chemistry, 07.10.2019 08:02 ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions

Mathematics, 06.11.2020 21:10

History, 06.11.2020 21:10

History, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Biology, 06.11.2020 21:10

Social Studies, 06.11.2020 21:10


Health, 06.11.2020 21:10

Biology, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Mathematics, 06.11.2020 21:10

Social Studies, 06.11.2020 21:10


English, 06.11.2020 21:10

English, 06.11.2020 21:10

History, 06.11.2020 21:10

Chemistry, 06.11.2020 21:10


Mathematics, 06.11.2020 21:10

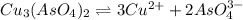
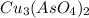 will be given by:
will be given by: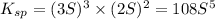
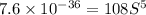
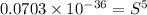
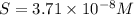
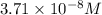 .
.

