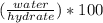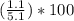Chemistry, 05.02.2020 13:01 andregijoe41
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. what is the percentage by mass of water in the original hydrate?
6.11%
21.6%
27.5%
78.4%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. w...
Questions

Advanced Placement (AP), 05.05.2020 18:32

Mathematics, 05.05.2020 18:32

Computers and Technology, 05.05.2020 18:32

History, 05.05.2020 18:32

Mathematics, 05.05.2020 18:32

Social Studies, 05.05.2020 18:32


English, 05.05.2020 18:32


Mathematics, 05.05.2020 18:33


Mathematics, 05.05.2020 18:33

Mathematics, 05.05.2020 18:33





Chemistry, 05.05.2020 18:33