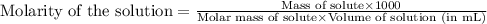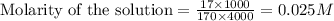Chemistry, 03.02.2020 00:49 hayleegahr
A4,000 ml solution of agno3 contains 17.00 g of solute in water. calculate the molarity (molar concentration) of the solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
A4,000 ml solution of agno3 contains 17.00 g of solute in water. calculate the molarity (molar conce...
Questions


Mathematics, 28.04.2021 23:20

Mathematics, 28.04.2021 23:20


Mathematics, 28.04.2021 23:20




Biology, 28.04.2021 23:20


Engineering, 28.04.2021 23:20



Mathematics, 28.04.2021 23:20

Mathematics, 28.04.2021 23:20

Mathematics, 28.04.2021 23:20



Mathematics, 28.04.2021 23:20

History, 28.04.2021 23:20