
Chemistry, 25.12.2019 15:31 sofiisabella10
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given off in a reaction. suppose after five trials of a chemical reaction, 200 kj of heat was taken in on average. what conclusion can be made about the reaction? a. it is endothermic
.b. it releases heat energy.
c. it is a combustion reaction.
d. it creates more than one product.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given...
a calorimeter is a device used to measure how much heat energy is taken in or given...
Questions

Mathematics, 25.11.2020 22:30

Physics, 25.11.2020 22:30

Biology, 25.11.2020 22:30


Mathematics, 25.11.2020 22:30



English, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40

Mathematics, 25.11.2020 22:40



Mathematics, 25.11.2020 22:40

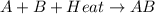
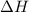 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.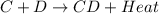 is an exothermic reaction.
is an exothermic reaction. 

