
Classifying reactions lab !
classifying reactions
safety reminder: wear safety glasses and use ammonia in a well-ventilated area. be sure to read science safety (link on page 1 of this lesson or page 872 in textbook)
objectives:
balance chemical equations (u4l1)
identify different types of reactions (u4l2)
describe evidence that a chemical reaction took place (u2l3)
day 1
part 1 reaction between iron and oxygen (record observations for 5 days)
materials:
small glass jar
dish with sides (a pie plate works well)
steel wool
water
procedure:
1. dip a piece of steel wool in vinegar for about 5 minutes to remove any coatings and then rinse it with water.
2. tape the piece of steel wool to the bottom of the jar.
3. fill the dish about halfway with water.
4. put the jar upside down in the dish.
5. observe the water levels in the dish and the jar and observe the steel wool in the jar for 5 days.
record your observations each day in the table below.
observations:
day|water level in dish (cm)|water level in jar (cm)|appearance of steel wool
1 | | |
2 | | |
3 | | |
4 | | |
5 | | |
analysis/conclusions:
1. balance the equation for the reaction between iron and oxygen.
+ →
2. classify the reaction that occurred between the iron and oxygen.
3. what evidence was there that a reaction took place?
part ii: reaction of hydrogen peroxide
materials:
hydrogen peroxide (1/3 cup)
small pieces of raw potato (yeast or beef liver may be substituted)
small bowl
procedure:
pour one third of a cup of hydrogen peroxide into a small bowl.
cut up several small pieces of raw potato and place them in the hydrogen peroxide.
3. record your observations.
observations:
appearance of potato and hydrogen peroxide combination
analysis/conclusion:
1. the potato (as well as yeast and beef liver) contains an enzyme called catalase, which breaks down hydrogen peroxide. balance the equation, if necessary, for the breakdown of hydrogen peroxide.
→ +
2. classify the reaction that occurred.
3. what evidence was there that a reaction was taking place?
day 2
part iii: reaction between zinc and acetic acid
materials:
a penny dated after 1983
metal file or coarse sandpaper
vinegar
procedure:
use a file or sandpaper to completely remove the copper from the edge of a penny. once the copper is removed you can see the silvery zinc that composes the core of the penny.
place the penny into a small jar of vinegar so that the penny sits upright. this will allow the hydrogen gas to readily escape and increase the rate of reaction.
allow the container to stand undisturbed for 30 minutes.
while the mixture is standing, complete steps 1 and 2 of part iv of the lab. be sure to record on the next page your observations for part iii after 30 minutes.
observations:
appearance of penny in vinegar after 30 minutes
analysis/conclusions:
1. balance the equation for the reaction between zinc and acetic acid.
+ → (ch3coo)2 +
2. classify the reaction between zinc and acetic acid.
3. what evidence was there that a reaction was taking place?
part iv: reaction between magnesium sulfate and ammonia
materials:
epsom salts (mgso4),
ammonia (nh4oh)
small jar or cup
measuring cup
procedure:
dissolve 1 teaspoon of epsom salts in a half cup of water.
add 2 teaspoons of ammonia to the epsom salts solution and stir thoroughly. record your observations.
allow the mixture to stand for 30 minutes and record your observations.
observations:
appearance after initial mixing
appearance after 30 minutes 
analysis/conclusions:
1. balance the equation for the reaction between magnesium sulfate and ammonia.
(2 points)
+ → )2so4 + (oh)2
2. classify the reaction between magnesium sulfate and ammonia.
3. what evidence was there to indicate that the epsom salts and ammonia reacted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 22:30
Write the formula for the conjugate base acid of hexanoic acid
Answers: 1
You know the right answer?
Classifying reactions lab !
classifying reactions
safety reminder: wear safety g...
classifying reactions
safety reminder: wear safety g...
Questions


Mathematics, 05.06.2021 21:10


Chemistry, 05.06.2021 21:10


Mathematics, 05.06.2021 21:10

Mathematics, 05.06.2021 21:10

Engineering, 05.06.2021 21:10

Mathematics, 05.06.2021 21:10






Mathematics, 05.06.2021 21:10

Computers and Technology, 05.06.2021 21:10

English, 05.06.2021 21:20

Social Studies, 05.06.2021 21:20

Mathematics, 05.06.2021 21:20

Chemistry, 05.06.2021 21:20


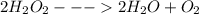
 .
.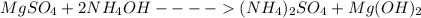 .
.

