
Chemistry, 31.10.2019 09:31 chloeozenghar1
What is the value for δsºreaction for the following reaction, given the standard entropy values?
al2o3(s) + 3h2(g) → 2al(s) + 3h2o(g)
substance sº(j/mol*k)
al2o3(s) 51
h2(g) 131
al(s) 28
h2o(g) 189

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
What is the value for δsºreaction for the following reaction, given the standard entropy values?
Questions

Business, 28.04.2021 02:50


History, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50



Chemistry, 28.04.2021 02:50


Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50


Mathematics, 28.04.2021 02:50


Mathematics, 28.04.2021 02:50

Biology, 28.04.2021 02:50


Mathematics, 28.04.2021 02:50


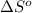 of the reaction is
of the reaction is 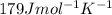
![\Delta S_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0354/1276/ec939.png)
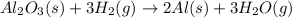
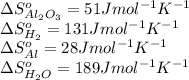
![\Delta S^o_{rxn}=[(2\times \Delta S^o_{H_2O})+(3\times \Delta S^o_{H_2O})]-[(1\times \Delta S^o_{Al_2O_3})+(3\times \Delta S^o_{H_2})]](/tpl/images/0354/1276/e83b3.png)
![\Delta S^o=[(2\times 28)+(3\times 189)]-[(1\times 51)+(3\times 131)]=179Jmol^{-1}K^{-1}](/tpl/images/0354/1276/23814.png)



