
The illustration depicts possible routes of collisions in the reaction ch₂ch₂ + hcl ch₃ch₂cl. which of the following statements is true?
a. the chlorine atom does not participate in the reaction.
b. the hydrogen atom does not participate in the reaction.
c. the speed of the collision is essential.
d. the orientation of the reactants is critical.
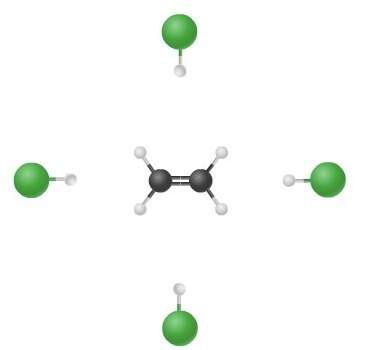

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

You know the right answer?
The illustration depicts possible routes of collisions in the reaction ch₂ch₂ + hcl ch₃ch₂cl. which...
Questions




Business, 16.11.2020 17:50

History, 16.11.2020 17:50


Social Studies, 16.11.2020 17:50


Mathematics, 16.11.2020 17:50


Mathematics, 16.11.2020 17:50











