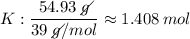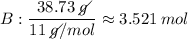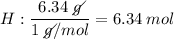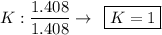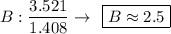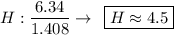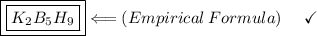Chemistry, 28.09.2019 00:00 romeojose2005
Find the empirical formula of a compound which contains 54.93% potassium, 38.73% boron and 6.34% hydrogen.
a.
kbh
b.
kb2h4
c.
kb3h9
d.
k2b5h9

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Find the empirical formula of a compound which contains 54.93% potassium, 38.73% boron and 6.34% hyd...
Questions




Mathematics, 31.08.2020 02:01



Medicine, 31.08.2020 02:01





Mathematics, 31.08.2020 02:01


Health, 31.08.2020 02:01

Advanced Placement (AP), 31.08.2020 02:01


Advanced Placement (AP), 31.08.2020 02:01



Mathematics, 31.08.2020 02:01