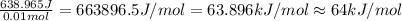Chemistry, 28.01.2020 03:31 uwunuzzles
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when a 200.0 ml volume of 0.100 m agno3 solution is mixed with a 100.0 ml sample of 0.100 m nacl solution, the temperature in the calorimeter rises to 25.16°c. determine the dh°rxn, in units of kj/mol agcl. assume that the density and heat capacity of the solutions is the same as that of water.
a. -78 kj/mol agcl
b. -25 kj/mol agcl
c. -64 kj/mol agcl
d. -32 kj/mol agcl
e. -59 kj/mol agcl

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when...
Questions

Biology, 04.01.2022 15:50




Mathematics, 04.01.2022 15:50





Mathematics, 04.01.2022 15:50


Chemistry, 04.01.2022 16:00


Mathematics, 04.01.2022 16:00

Chemistry, 04.01.2022 16:00


SAT, 04.01.2022 16:00

Social Studies, 04.01.2022 16:00

SAT, 04.01.2022 16:00

SAT, 04.01.2022 16:00

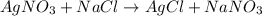
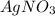 solution ,m'=
solution ,m'=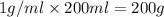
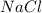 solution ,m''=
solution ,m''=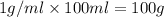
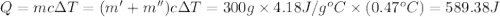
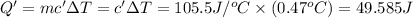
 =49.585 J+589.38 J=638.965 J
=49.585 J+589.38 J=638.965 J