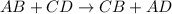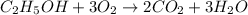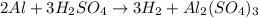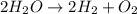Chemistry, 19.09.2019 01:00 seider7997
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 → 2co2 + 3h2o b) rb2o + cu(c2h3o2)2 → 2rbc2h3o2 + cuo c) 2al + 3h2so4 → 3h2 + al2(so4)3 eliminate d) 2h2o → 2h2 + o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 →...
Questions

Social Studies, 22.07.2021 03:30


Mathematics, 22.07.2021 03:40



History, 22.07.2021 03:40

Mathematics, 22.07.2021 03:40



Mathematics, 22.07.2021 03:40

Social Studies, 22.07.2021 03:40


English, 22.07.2021 03:40




Mathematics, 22.07.2021 03:40