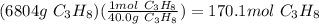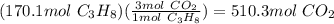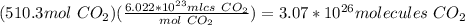Chemistry, 02.09.2019 07:00 kaitlynhess
Propane is used as a fuel in most gas grills to cook food on during the warm summer months. when the propane is burned the following reaction takes place:
c3h8(l) + 5o2(g) = 3co2(g) + 4h20(g)
a standard propane tank contains 6804 g of propane. determine how many molecules of carbon dioxide gas are released into the atmosphere with an entire tank of propane is burned. in your answer be sure to: describe the type of chemical reaction the propane undergoes, calculate the number of moles of propane used in the reaction, explain the mole ratio between propane and carbon dioxide in this reaction, calculate the number of moles of carbon dioxide produced, and calculate the number of molecules of carbon dioxide produced.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
Propane is used as a fuel in most gas grills to cook food on during the warm summer months. when the...
Questions


Mathematics, 30.06.2021 01:00






Mathematics, 30.06.2021 01:00



Mathematics, 30.06.2021 01:00

Mathematics, 30.06.2021 01:00

Mathematics, 30.06.2021 01:00

Mathematics, 30.06.2021 01:00



Biology, 30.06.2021 01:00


Business, 30.06.2021 01:00

Mathematics, 30.06.2021 01:00