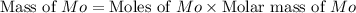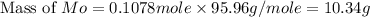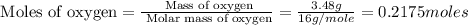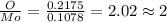Chemistry, 19.09.2019 03:30 Kekkdkskdkdk
A12.95 g sample of mo2o3(s) is converted completely to another molybdenum oxide by adding oxygen. the new oxide has a mass of 13.82 g. add subscripts below to correctly identify the empirical formula of the new oxide.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1
You know the right answer?
A12.95 g sample of mo2o3(s) is converted completely to another molybdenum oxide by adding oxygen. th...
Questions

Biology, 27.01.2020 16:31


English, 27.01.2020 16:31




Mathematics, 27.01.2020 16:31

History, 27.01.2020 16:31

History, 27.01.2020 16:31



Biology, 27.01.2020 16:31


Mathematics, 27.01.2020 16:31





English, 27.01.2020 16:31

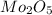 .
.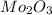 .
.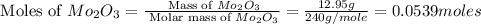
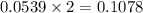 mole 'Mo'
mole 'Mo'