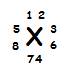
How to draw an. electron dot diagram. 1 write the chemical aymbo of the atom.2. determine the number of valence electron the atom has.3. place first two dot on the right side of the symbol.4. place the remaining dots around the symbol( on the bottom. the left side, and on top) 5. there should not be any more than two dots on either side .top. or bottom, and no electron should be left over. its my first time doing these .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
How to draw an. electron dot diagram. 1 write the chemical aymbo of the atom.2. determine the number...
Questions



Physics, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30


World Languages, 07.07.2019 00:30

Physics, 07.07.2019 00:30


Physics, 07.07.2019 00:30

History, 07.07.2019 00:30



English, 07.07.2019 00:30




History, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30

History, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30