
Chemistry, 17.10.2019 04:30 Jacobolobo7
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthalpy for the decomposition of 1 mole of mgo(s) into mg(s) and o2(g)?
consider this combination reaction:
what is the enthalpy for the decomposition of 1 mole of into and ?
-1204 kj/mol
602 kj/mol
1204 kj/mol
-602 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
Questions


Biology, 31.03.2021 18:00



Mathematics, 31.03.2021 18:00

Health, 31.03.2021 18:00


Mathematics, 31.03.2021 18:00





Mathematics, 31.03.2021 18:00





Mathematics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00

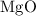 is
is 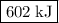 .
.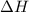 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.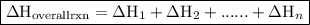
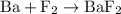
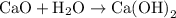
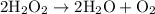
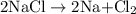
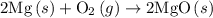
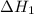 is
is 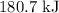 .
.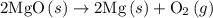
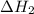 .
.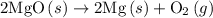 ......(2)
......(2)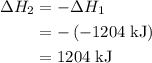
 dissociates to give two moles of
dissociates to give two moles of 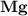 and one mole of
and one mole of 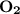 and therefore the enthalpy for the decomposition of one mole of is as follows:
and therefore the enthalpy for the decomposition of one mole of is as follows: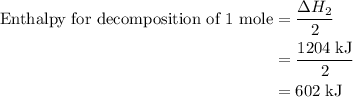
 .
.


