
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bubbles as a reaction takes place. you then weigh the resulting solution and find that it has a mass of 64.96 g . the relevant equation is
caco3(s)+2hcl(aq)→h2o(l)+co2(g)+cac l2(aq)
assuming no other reactions take place, what mass of co2 was produced in this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1
You know the right answer?
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bu...
Questions





Computers and Technology, 16.01.2020 00:31

History, 16.01.2020 00:31

Mathematics, 16.01.2020 00:31


English, 16.01.2020 00:31



Chemistry, 16.01.2020 00:31



English, 16.01.2020 00:31



Mathematics, 16.01.2020 00:31


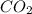 produced will be, 6.16 grams.
produced will be, 6.16 grams. = 14 g
= 14 g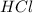 = 56.70 g
= 56.70 g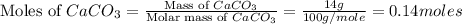
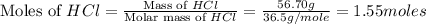
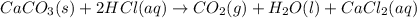
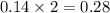 moles of
moles of 



