The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many...

Chemistry, 30.08.2019 22:30 Lovely3719
The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many moles of co2 are produced when 35.0 mol c2h2 react completely? how do you get 35.0 mol out of c2h2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Questions

Mathematics, 21.09.2019 07:30

History, 21.09.2019 07:30


English, 21.09.2019 07:30


History, 21.09.2019 07:30



Physics, 21.09.2019 07:30

History, 21.09.2019 07:30

Biology, 21.09.2019 07:30


English, 21.09.2019 07:30

English, 21.09.2019 07:30




Chemistry, 21.09.2019 07:30

Social Studies, 21.09.2019 07:30

Mathematics, 21.09.2019 07:30

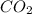 produced are, 70 moles
produced are, 70 moles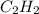 = 35 mole
= 35 mole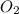 = 84 mole
= 84 mole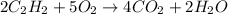
![\farc{4}{2]\times 35=70](/tpl/images/0213/2096/3d3b6.png) moles of carbon dioxide
moles of carbon dioxide

