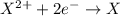Chemistry, 23.10.2019 08:00 lezapancakes13
An ion with a charge of +3 would most likely be an element in group
a.
1
b.
16
c.
18
d.
13
2.
an element in group 2 would most likely form what kind of bond?
a.
covalent
b.
ionic
c.
polar covalent
d.
hydrogen
3.
when an ion with a charge of +2 gains two electrons, it no longer is an ion.
a.
true
b.
false

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
You know the right answer?
An ion with a charge of +3 would most likely be an element in group
a.
1
b....
a.
1
b....
Questions




Biology, 15.01.2021 22:50

History, 15.01.2021 22:50


World Languages, 15.01.2021 22:50


Mathematics, 15.01.2021 22:50


Chemistry, 15.01.2021 22:50

Computers and Technology, 15.01.2021 22:50




Mathematics, 15.01.2021 22:50

Mathematics, 15.01.2021 22:50

Mathematics, 15.01.2021 22:50

Computers and Technology, 15.01.2021 22:50

 etc..
etc..
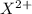 ions and thus tends to form ionic bonds.
ions and thus tends to form ionic bonds.