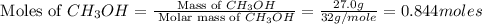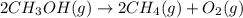Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amo...

Chemistry, 25.10.2019 18:43 samafeggins2
Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amount of heat transferred when 27.0g of ch3oh(g) is decomposed by this reaction at constant pressure.
if someone could me with the steps i can figure it out on my own, you so much

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2



Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Questions




Mathematics, 22.08.2020 19:01


History, 22.08.2020 19:01


Mathematics, 22.08.2020 19:01

Mathematics, 22.08.2020 19:01



Mathematics, 22.08.2020 19:01

Biology, 22.08.2020 19:01

Mathematics, 22.08.2020 19:01




Biology, 22.08.2020 19:01


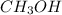 = 27.0 g
= 27.0 g