
Chemistry, 06.10.2019 00:30 twistedhyperboles
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bicarbonate (nahco3) reacting with one mole of hydrochloric acid (hcl) to produce one mole of carbonic acid (h2co3), and one mole of sodium chloride (nacl). using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol of nahco3 and 8 mol of hcl.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bica...
Questions



Arts, 10.10.2019 05:30




Computers and Technology, 10.10.2019 05:30



Biology, 10.10.2019 05:30



Business, 10.10.2019 05:30





Mathematics, 10.10.2019 05:30


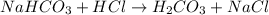
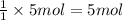 of HCl
of HCl

