
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?
each bond in the molecule is only moderately polar covalent.
the molecule is too small to be polar.
the polar bonds are oriented in opposite directions.
the carbon atom has two unshared pairs of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?...
Questions


Mathematics, 27.08.2019 08:30

English, 27.08.2019 08:30

Mathematics, 27.08.2019 08:30

Biology, 27.08.2019 08:30

Biology, 27.08.2019 08:30

Chemistry, 27.08.2019 08:30

English, 27.08.2019 08:30


Mathematics, 27.08.2019 08:30

English, 27.08.2019 08:30

Mathematics, 27.08.2019 08:30

History, 27.08.2019 08:30

Mathematics, 27.08.2019 08:30


English, 27.08.2019 08:30



History, 27.08.2019 08:30

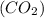 contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.
contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.

