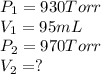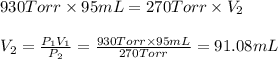Chemistry, 29.08.2019 17:30 Randy11111
Agas has a volume of 95 ml at a pressure of 930 torr. what volume will the gas occupy if the pressure is increased to 970 torr and the temperature remains constant

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Agas has a volume of 95 ml at a pressure of 930 torr. what volume will the gas occupy if the pressur...
Questions


Mathematics, 18.10.2019 08:10

Mathematics, 18.10.2019 08:10

Biology, 18.10.2019 08:10


Mathematics, 18.10.2019 08:10

Mathematics, 18.10.2019 08:10

Social Studies, 18.10.2019 08:10


Mathematics, 18.10.2019 08:20

English, 18.10.2019 08:20

Biology, 18.10.2019 08:20


Mathematics, 18.10.2019 08:20






Chemistry, 18.10.2019 08:20

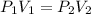 (at constant temperature)
(at constant temperature)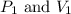 are initial pressure and volume.
are initial pressure and volume.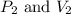 are final pressure and volume.
are final pressure and volume.