
Chemistry, 19.04.2021 21:20 skrillex88
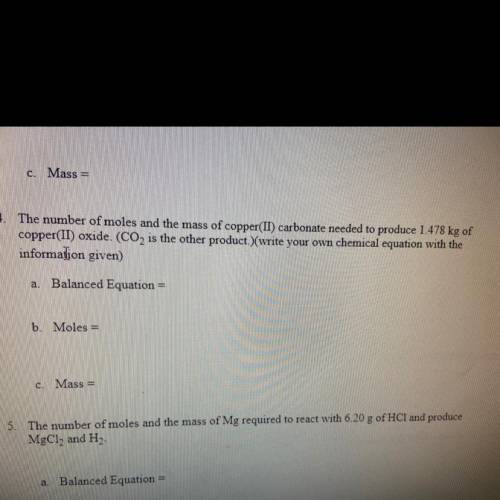
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two is the other products are your own chemical equation with the information given

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two...
Questions

Mathematics, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30



History, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30

History, 04.12.2020 21:30

Social Studies, 04.12.2020 21:30

Social Studies, 04.12.2020 21:30



Advanced Placement (AP), 04.12.2020 21:30


Health, 04.12.2020 21:30


Mathematics, 04.12.2020 21:30





