Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
...

Chemistry, 19.04.2021 21:00 shyshy1791
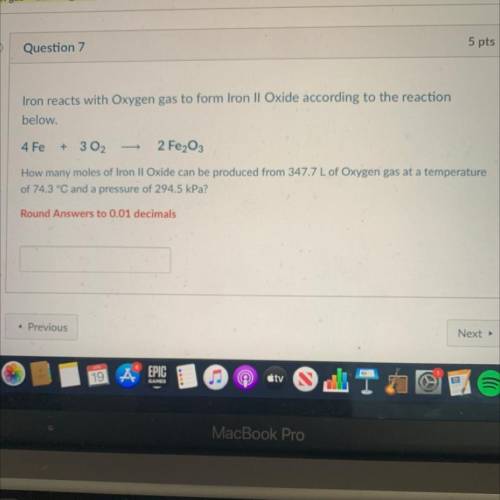
Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
+ 3 02
2 Fe2O3
How many moles of Iron II Oxide can be produced from 347.7 L of Oxygen gas at a temperature
of 74.3 °C and a pressure of 294.5 kPa?
Round Answers to 0.01 decimals

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Questions





Mathematics, 12.10.2020 21:01

History, 12.10.2020 21:01


English, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01


English, 12.10.2020 21:01


English, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01


English, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01




