
Chemistry, 19.04.2021 18:00 muncyemily
Calculate the number of water (H2O) molecules produced from the decomposition of 75.50 grams of Iron (III) hydroxide (Fe(OH)3).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
Calculate the number of water (H2O) molecules produced from the decomposition of 75.50 grams of Iron...
Questions


Advanced Placement (AP), 18.06.2020 22:57


Law, 18.06.2020 22:57


English, 18.06.2020 22:57


Spanish, 18.06.2020 22:57

English, 18.06.2020 22:57




Mathematics, 18.06.2020 22:57



Biology, 18.06.2020 22:57


Social Studies, 18.06.2020 22:57

Mathematics, 18.06.2020 22:57

Computers and Technology, 18.06.2020 22:57

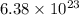 molecules of water are produced.
molecules of water are produced.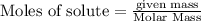
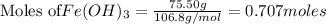
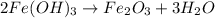
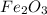 produce = 3 moles of
produce = 3 moles of 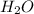
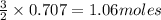 of
of 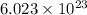 of particles.
of particles.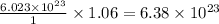 molecules
molecules

