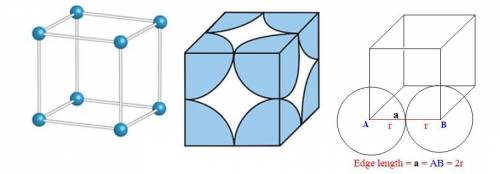
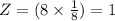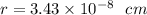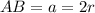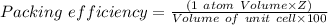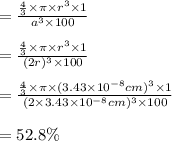The different unit cell types have a different packing efficiency. The simple cubic has the least efficient packing and the face-centered cubic has the most efficient packing (sometimes the face-centered cubic is called cubic closest packing). You can determine what percent of the unit cell is occupied by (1) determining the volume of the whole unit cell and (2) determining the volume of the occupied space by the atoms in the unit cell. Remember that the volume of a cube is V

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1
You know the right answer?
The different unit cell types have a different packing efficiency. The simple cubic has the least ef...
Questions


English, 18.09.2019 14:50


Biology, 18.09.2019 14:50


Mathematics, 18.09.2019 14:50

Chemistry, 18.09.2019 14:50








History, 18.09.2019 14:50

History, 18.09.2019 14:50



Biology, 18.09.2019 14:50

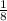 to both the cell unit
to both the cell unit