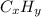Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
7. A certain hydrocarbon, CxHy, is burned (reacts with O2 gas) and produces 1.955 g of CO2 for every...
Questions


Social Studies, 19.03.2020 09:31

Mathematics, 19.03.2020 09:31




Biology, 19.03.2020 09:31





English, 19.03.2020 09:31

Mathematics, 19.03.2020 09:31






Biology, 19.03.2020 09:31

Chemistry, 19.03.2020 09:31